
Background: High-risk B- and T- cell Acute Lymphoblastic Leukemia (B/T-ALL) are aggressive lymphoid malignancies being treated with inhibitors targeting oncogenes and CAR-T cell-based immunotherapies. Despite advances in treatment, emergence of therapeutic resistance poses a clinical challenge in combating B/T-ALL. More recently, NK cells have emerged as attractive immunotherapies for cancers. Furthermore, NK therapies show promise for T-ALL, that has been particularly hard to treat with CAR-T cells.
Approach: To develop NK therapies against B/T-ALL, it is important to identify the phenotypic and functional deficiencies in NK cells of ALL patients. Therefore, using mass cytometry (CyTOF), we compared the profile of NK cells in bone marrow (BM) or peripheral blood (PB) of 20 high-risk ALL patients (13 B-ALL, 7 T-ALL, 9 pediatric and 11 adult) and 22 tissue-matched healthy donors. We validated our CyTOF results by conducting in silico cytometry (CIBERSORT) for NK subsets in an independent panel of 207 high-risk B-ALL patients from the Children's Oncology Group (COG) P9906 clinical trial. We then investigated whether distinct NK signatures identified by CyTOF and CIBERSORT in B/T-ALL correlate with risk features and predict clinical outcome.
Results: We observed a significant reduction in the frequency of total NK cells within the non-malignant immune fraction in B/T-ALL. Upon comparing the frequencies of specific NK subsets (CD56Brightand CD56Dim, we found that CD56Bright NK cells were increased in the BM (P=0.0138) but were reduced in PB (P=0.0285) of patients as compared to healthy donors. We then conducted CIBERSORT to estimate the relative proportions of NK cells with CD56Bright and CD56Dim molecular signatures in 207 COG B-ALL patients compared to 94 healthy donors. We found that frequencies of NK cells with CD56Bright molecular signature were increased in both BM and PB of ALL patients (P<0.0001). We conclude that majority of NK cells in B/T-ALL exhibit the molecular profile of CD56Bright NK cells, albeit their PB NK cells show reduced surface CD56.
Human NK cells mature from CD56Bright to CD56Dim stages. CD56Dim NK are more cytotoxic and produce less cytokines than CD56Bright NK cells. Enrichment of CD56Bright molecular signature in NK cells of B/T-ALL patients suggested an impairment in NK maturation and function in ALL. By comparing the abilities of PB NK cells in B/T-ALL and healthy donors to lyse K562 targets invitro, we found that NK cells in ALL patients are poorly cytolytic compared to healthy donors (P=0.0295). Upon stimulation with PMA/Ionomycin, we observed an increase in the frequencies of GM-CSF+ and TNF-α+ cells in total NK and NK subsets in BM and PB of B/T-ALL patients compared to healthy donors (all P<0.05). Surprisingly, the natural cytotoxicity receptor NKp46 was increased in stimulated NK subsets of B/T-ALL patients compared to healthy controls (P<0.01). We conclude that poorly cytotoxic NK cells with CytokineHigh NKp46High activated phenotype (NKActiv) are enriched in B/T-ALL, while normal NK phenotype (NKRest) is suppressed.
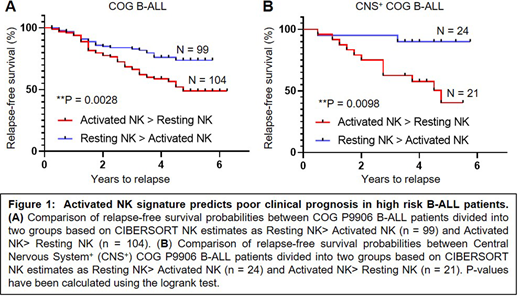
Finally, we investigated whether relative frequencies of NKActiv and NKRest cells can predict clinical outcome in high-risk ALL. To this end, we used CIBERSORT to estimate proportions of NK cells with activated (NKActiv) and resting (NKRest) molecular signatures in 207 COG B-ALL patients. We then separated patients into 2 groups as NKActiv> NKRest and NKRest> NKActiv, and compared their relapse-free survival (RFS) probabilities (Fig.1). We found that patients with higher NKActiv cells had shorter RFS than those with higher NKRest cells (P=0.0028). Furthermore, we found that relative proportions of activated and resting NK cells independently predict clinical outcome within a poorly prognostic subset of patients with Central Nervous System involvement (CNS+, P=0.0098). These preliminary findings suggest that higher levels of activated NK cells in B-ALL may be associated with poor clinical prognosis. We are validating these results in B/T-ALL from our CyTOF studies.
Conclusion: We find that impairment of NK maturation in high-risk human B/T-ALL results in the accumulation of NK cells with a poorly cytotoxic but hyperactivated cytokine-producing phenotype, that positively correlates with poor clinical prognosis. Our data suggest that restoring NK homeostasis would be an attractive strategy for treating high-risk B/T-ALL.
Marcucci:Novartis: Speakers Bureau; Abbvie: Speakers Bureau; Iaso Bio: Membership on an entity's Board of Directors or advisory committees; Takeda: Other: Research Support (Investigation Initiated Clinical Trial); Merck: Other: Research Support (Investigation Initiated Clinical Trial); Pfizer: Other: Research Support (Investigation Initiated Clinical Trial).
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal